Abeta 1-42 ELISA 试剂盒
-
- 抗原 See all Abeta 1-42 ELISA试剂盒
- Abeta 1-42 (Amyloid beta 1-42 (Abeta 1-42))
-
适用
- 人
- 检测方法
- Colorimetric
- 实验类型
- Sandwich ELISA
- 检测范围
- 4.688 pg/mL - 300 pg/mL
- 最低检测浓度
- 4.688 pg/mL
- 应用范围
- ELISA
- 原理
- For quantitative detection of Aβ42 in serum, plasma, tissue homogenates.
- 样品类型
- Plasma, Serum, Tissue Homogenate
- Analytical Method
- Quantitative
- 特异性
- This assay has high sensitivity and excellent specificity for detection of Aβ42. No significant cross-reactivity or interference between Aβ42 and analogues was observed. Note: Limited by current skills and knowledge, it is difficult for us to complete the cross-reactivity detection between Aβ42 and all the analogues, therefore, cross reaction may still exist.
- 灵敏度
- 2.813 pg/mL
- 组件
-
- Pre-coated, ready to use 96-well strip plate
- Plate sealer for 96 wells
- Lyophilized Standard
- Sample/Standard Dilution Buffer
- Biotin-labeled Antibody (Concentrated)
- Antibody Dilution Buffer
- HRP-Streptavidin Conjugate (SABC)
- SABC Dilution Buffer
- TMB Substrate
- Stop Solution
- Wash Buffer (25 x concentrate)
- Plate Sealer
- Instruction manual
- 试剂未包括
-
- Microplate reader (wavelength:450nm)
- 37 °C incubator
- Automated plate washer
- Precision single and multi-channel pipette and disposable tips
- Clean tubes and Eppendorf tubes
- Deionized or distilled water
-
-
- 样本量
- 100 μL
- 板类型
- Pre-coated
- 实验流程
-
- Set standard, test samples, control (blank) wells on the pre-coated plate respectively, and then, records their positions.
- Add 100ul standard or sample to each well and incubate at room temperature for 120 minutes.
- Aspirate and wash plates 2 times.
- Add 100ul Biotin-labeled antibody working solution to each well and incubate at room temperature for 60 minutes.
- Aspirate and wash plates 3 times.
- Add 100ul SABC Working Solution into each well and incubate at room temperature for 30 minutes.
- Aspirate and wash plates 5 times.
- Add 90 µL TMB Substrate Solution. Incubate 10-20 minutes at 37 °C.
- Add 50 µL Stop Solution. Read at 450nm immediately and calculation.
- 试剂准备
-
- Bring all reagents and samples to room temperature for 20 minutes before use.
- Wash Buffer: If crystals have formed in the concentrate, you can warm it with 40 °C water bath Concentrated Wash Buffer into 750 mL Wash Buffer with deionized or distilled water. Put unused solution back at 2-8 °C.
- Standards:
- Add 1 mL Sample Dilution Buffer into one Standard tube (labeled as zero tube), keep the tube at room temperature for 10 minutes and mix them thoroughly. Note: If the standard tube concentration higher than the range of the kit,please dilute it and labeled as zero tube.
- Label 7 EP tubes with 1/2, 1/4, 1/8, 1/16, 1/32, 1/64 and blank respectively. Add 0.3 mLof the Sample Dilution Buffer into each tube. Add 0.3 mLof the above Standard solution (from zero tube) into 1st tube and mix them thoroughly. Transfer 0.3 mL from 1st tube to 2nd tube and mix them thoroughly. Transfer 0.3 mL from 2nd tube to 3rd tube and mix them thoroughly, and so on. Sample Dilution Buffer was used for the blank control. Note: It is best to use Standard Solutions within 2 hours.
- Preparation of Biotin-labeled Antibody Working Solution:
Prepare it within 1 hour before experiment.- Calculate required total volume of the working solution: 0.1ml/well x quantity of wells. (Allow 0.1-0.2 mLmore than the total volume.)
- Dilute the Biotin-detection antibody with Antibody Dilution Buffer at 1:100 and mix them thoroughly. (i.e. Add 1 µL Biotin-labeled antibody into 99 µL Antibody Dilution Buffer.)
- Preparation of HRP-Streptavidin Conjugate (SABC) Working Solution:
Prepare it within 30 minutes before experiment.- Calculate required total volume of the working solution: 0.1ml/well x quantity of wells. (Allow 0.1-0.2 mLmore than the total volume.)
- Dilute the SABC with SABC Dilution Buffer at 1:100 and mix them thoroughly. (i.e. Add 1 µL of SABC into 99 µL of SABC Dilution Buffer.)
- 样品制备
-
- It is recommended to use fresh samples without long storage, otherwise protein degradation and denaturation may occur in these samples, leading to false results. Samples should therefore be stored for a short period at 2 - 8 °C or aliquoted at -20 °C (≤1 month) or -80 °C (≤ 3 months). Repeated freeze-thaw cycles should be avoided. Prior to assay, the frozen samples should be slowly thawed and centrifuged to remove precipitates.
- If the sample type is not specified in the instructions, a preliminary test is necessary to determine compatibility with the kit.
- If a lysis buffer is used to prepare tissue homogenates or cell culture supernatant, there is a possibility of causing a deviation due to the introduced chemical substance. The recommended dilution factor is for reference only.
- Please estimate the concentration of the samples before performing the test. If the values are not in the range of the standard curve, the optimal sample dilution for the particular experiment has to be determined.
Note:The user should estimate the concentration of target protein in the test sample, and select a proper dilution factor to make the diluted target protein concentration fall in the optimal detection range of the kit. Dilute the sample with the provided dilution buffer, and several trials may be necessary. The test sample must be well mixed with the dilution buffer. And also standard curves and sample should be making in pre-experiment. If samples with very high concentrations, dilute samples with PBS first and then dilute the samples with Sample Dilution. The matrix components in the sample will affect the test results, which it need to be diluted at least 1/2 with Sample Dilution Buffer before testing! - 实验流程
-
Washing
Manual: Discard the solution in the plate without touching the side walls. Clap the plate on absorbent filter papers or other absorbent material. Fill each well completely with 350 µL wash buffer and soak for 1 to 2 minutes, then aspirate contents from the plate, and clap the plate on absorbent filter papers or other absorbent material.
Automatic: Aspirate all wells, and then wash plate with 350 µL wash buffer. After the final wash, invert plate, and clap the plate on absorbent filter papers or other absorbent material. It is recommended that the washer shall be set for soaking 1 minute. (Note: set the height of the needles, be sure the fluid can be sipped up completely)When diluting samples and reagents, they must be mixed completely and evenly. Before adding TMB into wells, equilibrate TMB Substrate for 30 minutes at 37 °C. It is recommended to plot a standard curve for each test.
- Set standard, test samples (diluted at least 1/2 with Sample Dilution Buffer), control (blank) wells on the pre-coated plate respectively, and then, records their positions. It is recommended to measure each standard and sample in duplicate.
- Prepare Standards: Aliquot 100 µL of zero tube, 1sttube, 2ndtube, 3rdtube, 4thtube, 5thtube, 6thtube and Sample Dilution Buffer (blank) into the standard wells.
- Add Samples: Add 100 µL of properly diluted sample into test sample wells.
- Incubate: Seal the plate with a cover and incubate at room temperature for 120 minutes.
- Wash: Remove the cover and discard the plate content, and wash plate 2 times with Wash Buffer. Do NOT let the wells dry completely at any time.
- Biotin-labeled Antibody: Add 100ul Biotin-labeled antibody working solution into above wells (standard, test sample and blank wells). Add the solution at the bottom of each well without touching the sidewall, cover the plate and incubate at room temperature for 60 minutes.
- Wash: Remove the cover, and wash plate 3 times with Wash Buffer, and let the Wash Buffer stay in the wells for 1-2 minutes each time.
- HRP-Streptavidin Conjugate (SABC): Add 100ul of SABC Working Solution into each well, cover the plate and incubate at room temperature for 30 minutes.
- Wash: Remove the cover and wash plate 5 times with Wash Buffer, and let the wash buffer stay in the wells for 1-2 minutes each time.
- TMB Substrate: Add 90ul TMB Substrate into each well, cover the plate and incubate at 37°C in dark within 10-20 minutes. (Before adding TMB into wells, equilibrate TMB Substrate for 30 minutes at 37°C.). (Note: The reaction time can be shortened or extended according to the actual color change, but not more than 30 minutes. You can terminate the reaction when apparent gradient appeared in standard wells.)
- Stop: Add 50 µL Stop Solution into each well. The color will turn yellow immediately. The adding order of Stop Solution should be as the same as the TMB Substrate Solution.
- OD Measurement: Read the O.D. absorbance at 450nm in Microplate Reader immediately after adding the stop solution.
Note: If the samples measured were diluted, multiply the dilution factor to the concentrations from interpolation to obtain the concentration before dilution.
- 实验精密度
-
Intra-Assay: CV<8%
Inter-Assay: CV<10% - 限制
- 仅限研究用
-
- by
- Prof. Merighi, Laboratory of Neurobiology, Department of Veterinary Sciences, University of Turin
- No.
- #104609
- 日期
- 2024.09.17
- 抗原
- Abeta 1-42
- Lot Number
- FN240621
- Method validated
- ELISA
- Positive Control
-
- Negative Control
blank control
- Notes
Passed.
- Primary Antibody
- Secondary Antibody
- Full Protocol
- Sample analyzed: IDE activator-treated 7PA2 cells (passage #6). A total of 18 samples were used. Observations were obtained in triplicated samples of 6 different treatment groups, and their average values were taken as the response variable.
- Cell culture and treatment protocol:
- 3 x 10^5 7PA2 cells were seeded into 6-well plates and grown in Dulbecco’s modified medium with 10% fetal bovine serum, 100 units/ml penicillin, 100 µg/ml streptomycin, 200 µg/ml G418 (gentamycin), and 2 mM L-glutamine.
- When cells reached 90-95% confluency, the old media was aspirated, and cells were treated with 50 µM of IDE activators dissolved in a serum-free media.
- Control cells were treated with DMSO since the solvent of the chemicals was DMSO (not exceeding 0.5% of the total volume of the media).
- Cells were incubated for 72 hours at 37°C in a 5% CO2 incubator.
- Sample preparation:
- Cells were collected by scraping in PBS and centrifuged at 4°C, 10000 rpm for 10 min.
- The supernatant was removed, and 120 µl of lysis buffer (KCl 150 mM, HEPES 20 mM, MgCl2 5 mM, and DTT 0.5 mM) was added to the pellet.
- Tubes with samples were kept in the ice bucket for 15 min, and cells were disrupted by pipetting.
- Tubes were centrifuged at 4°C, 10000 rpm for 10 min.
- The supernatant was transferred into a new tube, and a portion of the supernatant was used for protein determination.
- The protein amount was determined by BCA assay, the total protein amount was calculated based on the albumin standard curve, and 100 µl of lysate was used for ELISA assay.
- Reagent preparation:
- All reagents and samples were brought to ambient temperature for 20 minutes before use.
- Wash buffer:
- The washing buffer might have crystals; to melt them down, it was kept in the water bath at 40℃.
- To prepare the washing buffer, 2 mL of 25x wash buffer was mixed with 48 mL of DW.
- The unused solution was kept at 4 °C.
- Standards:
- 1 mL of sample dilution buffer was added into one standard tube (labeled as a 0 tube); it was kept at room temperature for 10 minutes and mixed thoroughly.
- Seven tubes were labeled from 1 to 7.
- 300 µL of sample dilution buffer was added into each tube.
- 300 µL of the standard was transferred from the 0 tube to the 1st tube and mixed thoroughly.
- 300 µL of sample from the 1st tube was added to the 2nd tube and mixed thoroughly.
- This serial dilution was repeated until the 7th tube, and we got samples diluted at 1/2, 1/4, 1/8, 1/16, 1/32, and 1/64.
- Sample dilution buffer was added to the 7th tube and used as a blank.
- Biotin-labeled antibody working solution:
- The solution was prepared 1 hour before usage.
- The biotin-detection antibody was diluted with antibody dilution buffer at a 1:100 ratio.
- The mixture was prepared for 35 samples (1 extra for volume loss).
- 35 µL of biotin-labeled antibody was added to 3465 µL of antibody dilution buffer and mixed thoroughly.
- HRP-Streptavidin Conjugate Working Solution (SABC):
- The solution was prepared 30 minutes before usage.
- SABC was diluted with SABC dilution buffer at a 1:100 ratio.
- The mixture was prepared for 35 samples (1 extra for volume loss).
- 35 µL of SABC antibody was added to 3465 µL of SABC dilution buffer and mixed thoroughly.
- Assay procedure:
- Standards, test samples, and control (blank) were set on the pre-coated plate, respectively, and their positions were recorded.
- 100 µL of standard, sample, and blank were loaded into designated wells.
- The plate was sealed with a cover and incubated at room temperature for 120 minutes by slightly shaking at 300 rpm.
- The cover was removed, and the plate content was discarded by aspiration in a low vacuum pressure. Each well was washed twice with 300 µL of washing buffer.
- 100 µL of biotin-labeled antibody working solution was added into wells, and the plate was covered and incubated at room temperature for 60 minutes by slightly shaking at 300 rpm.
- The cover was removed, and plate content was discarded by aspiration in a low vacuum pressure. Each well was washed three times with 300 µL of washing buffer. Washing buffer stayed in the wells for 1-2 minutes each time.
- 100 µL of SABC working solution was added into each well; the plate was covered and incubated at room temperature for 30 minutes at 300 rpm.
- The cover was removed, and plate content was discarded by aspiration in a low vacuum pressure. Each well was washed five times with 300 µL of washing buffer. Washing buffer stayed in the wells for 1-2 minutes each time.
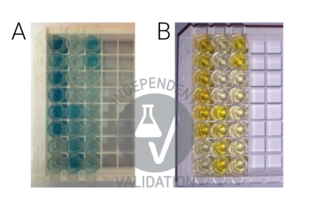
- Before adding TMB into wells, it was kept at 37°C for 30 minutes for equilibration. 90 µL of TMB substrate was added into each well; the plate was covered and incubated at 37°C in a dark place (the plate was wrapped with aluminum foil) for 20 minutes. The blue color was developed in the wells.
- The reaction was terminated by adding 50 µL of stop solution. The color turned yellow immediately. The adding order of the Stop solution was the same as the loading order of the TMB substrate solution.
- The plate was read immediately by a microplate reader at 450 nm wavelength.
- Calculation:
- Data analysis was performed in GraphPad Prism 10.3.1 software.
- Relative O.D.450 for samples was calculated using the following equation: The relative O.D.450 = The O.D.450 of each well - The O.D.450 of the blank well.
- The standard curve was plotted as the relative O.D.450 of each standard solution (Y) vs. the respective concentration of the standard solution (X) (4PL regression).
- The target concentration of the samples was interpolated from the standard curve.
- Experimental Notes
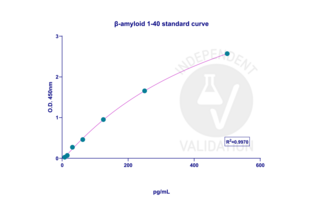
The aim of this experiment was the validation of ELISA kits, which determine the amount of β-amyloid 1-40 and 1-42 in biological samples. 7PA2 cells were used as a test sample after being treated with different IDE activators. 7PA2 cells stably express human APP751 bearing the V717F familial AD mutation and secrete significant amounts of APP products, and would be a perfect sample to validate β-amyloid ELISA kits.To generate a standard calibration curve, a wide range of concentrations were used for β-amyloid (1-40) and β-amyloid (1-42), between 7.8125-500 pg/ml for β-amyloid (1-40), and between 4.6875-300 pg/ml for β-amyloid (1-42). Blank O.D. values were subtracted from the standard sample and test O.D. values to remove the background signal. β-amyloid (1-40) displayed a higher background signal in comparison to the β-amyloid (1-42) ELISA kit, which was also the reason for the high deviation in CV% of the lowest concentrations of β-amyloid (1-40) standards, which means the usage of these two points might increase the variability of the actual amount of β-amyloid (1-40) in the test samples.
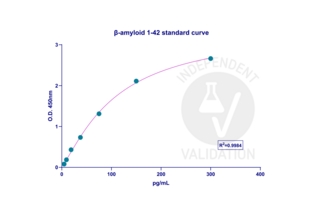
Standard sample concentrations of β-amyloid (1-42) showed low CV deviation, which led to building a good standard curve. A total of 18 test samples were analyzed (6 groups in triplicated samples), and the amount of β-amyloid (1-40) and β-amyloid (1-42) was determined by using the 4PL regression model curve by GraphPad Prism software. The amount of β-amyloid (1-40) and β-amyloid (1-42) in test samples was in the ranges of the calibration curve.
生效 #104609 (ELISA)![成功验证 '独立验证'标志]()
![成功验证 '独立验证'标志]() Validation Images
Validation Images![The standard calibration curve for β-amyloid (1-42) was generated with a 4PL regression model in GraphPad Prism 10.3.1. version. The standard curve was plotted as the relative O.D.450 of each standard solution (Y) vs. the respective concentration of the standard solution (X). The concentration range for generating the calibration curve was between 4.6875-300 pg/ml, and R2 was 0.9984.]() The standard calibration curve for β-amyloid (1-42) was generated with a 4PL regression model in GraphPad Prism 10.3.1. version. The standard curve was plotted as the relative O.D.450 of each standard solution (Y) vs. the respective concentration of the standard solution (X). The concentration range for generating the calibration curve was between 4.6875-300 pg/ml, and R2 was 0.9984.
The standard calibration curve for β-amyloid (1-42) was generated with a 4PL regression model in GraphPad Prism 10.3.1. version. The standard curve was plotted as the relative O.D.450 of each standard solution (Y) vs. the respective concentration of the standard solution (X). The concentration range for generating the calibration curve was between 4.6875-300 pg/ml, and R2 was 0.9984.
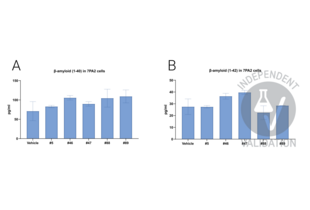
![Raw data and calculated amounts of β-amyloid (1-40) in 7PA2 cells treated with 5 different chemicals. The table represents the raw O.D. values of the standard solution, test samples, and blank. To remove the background signal, the O.D. value of the blank was subtracted from the O.D. values of standard samples and test samples. The calculation curve was generated by using 4PL regression in GraphPad Prism 10.3.1, and the β-amyloid (1-40) amount in test samples was calculated based on the standard curve. The obtained amounts were normalized to the total protein amount in the sample, which was detected by the BCA assay.]() Raw data and calculated amounts of β-amyloid (1-40) in 7PA2 cells treated with 5 different chemicals. The table represents the raw O.D. values of the standard solution, test samples, and blank. To remove the background signal, the O.D. value of the blank was subtracted from the O.D. values of standard samples and test samples. The calculation curve was generated by using 4PL regression in GraphPad Prism 10.3.1, and the β-amyloid (1-40) amount in test samples was calculated based on the standard curve. The obtained amounts were normalized to the total protein amount in the sample, which was detected by the BCA assay.
Raw data and calculated amounts of β-amyloid (1-40) in 7PA2 cells treated with 5 different chemicals. The table represents the raw O.D. values of the standard solution, test samples, and blank. To remove the background signal, the O.D. value of the blank was subtracted from the O.D. values of standard samples and test samples. The calculation curve was generated by using 4PL regression in GraphPad Prism 10.3.1, and the β-amyloid (1-40) amount in test samples was calculated based on the standard curve. The obtained amounts were normalized to the total protein amount in the sample, which was detected by the BCA assay.
![Raw data and calculated amounts of β-amyloid (1-42) in 7PA2 cells treated with 5 different chemicals. The table represents the raw O.D. values of the standard solution, test samples, and blank. To remove the background signal, the O.D. value of the blank was subtracted from the O.D. values of standard samples and test samples. The calculation curve was generated by using 4PL regression in GraphPad Prism 10.3.1, and the β-amyloid (1-42) amount in test samples was calculated based on the standard curve. The obtained amounts were normalized to the total protein amount in the sample, which was detected by the BCA assay.]() Raw data and calculated amounts of β-amyloid (1-42) in 7PA2 cells treated with 5 different chemicals. The table represents the raw O.D. values of the standard solution, test samples, and blank. To remove the background signal, the O.D. value of the blank was subtracted from the O.D. values of standard samples and test samples. The calculation curve was generated by using 4PL regression in GraphPad Prism 10.3.1, and the β-amyloid (1-42) amount in test samples was calculated based on the standard curve. The obtained amounts were normalized to the total protein amount in the sample, which was detected by the BCA assay.
Full Methods
Raw data and calculated amounts of β-amyloid (1-42) in 7PA2 cells treated with 5 different chemicals. The table represents the raw O.D. values of the standard solution, test samples, and blank. To remove the background signal, the O.D. value of the blank was subtracted from the O.D. values of standard samples and test samples. The calculation curve was generated by using 4PL regression in GraphPad Prism 10.3.1, and the β-amyloid (1-42) amount in test samples was calculated based on the standard curve. The obtained amounts were normalized to the total protein amount in the sample, which was detected by the BCA assay.
Full Methods -
- 储存条件
- 4 °C,-20 °C
- 储存方法
-
- For unopened kit: All the reagents should be kept according to the labels on vials. The Reference Standard and the 96-well stripe plate should be stored at -20 °C upon receipt while the other reagents should be stored at 4 °C.
- For used kit: When the kit is used, the remaining reagents need to be stored according to the above storage condition. Besides, please return the unused wells to the foil pouch containing the desiccant pack, and zip-seal the foil pouch.
- 有效期
- 6 months
-
- 抗原 See all Abeta 1-42 ELISA试剂盒
- Abeta 1-42 (Amyloid beta 1-42 (Abeta 1-42))
- 别名
- Amyloid Beta 42 (Abeta 1-42 产品)
- 背景
- Aβ42, Amyloid Beta 42, Aβ, 1-42, Aβ1-42
- UniProt
- P05067
-



 (1 validation)
(1 validation)



