Biosimilar Antibodies
Biosimilar antibodies have emerged as valuable tools for research purposes. These antibodies, derived from genetically engineered cells, mimic the structure and function of existing therapeutic antibodies, offering cost-effective alternatives for research applications. With advancements in recombinant DNA technology and protein expression systems, monoclonal biosimilar antibodies can be produced with high specificity and reproducibility, providing researchers with reliable reagents for various experiments. The availability of recombinant biosimilar research antibodies broadens the accessibility of research-grade antibodies, facilitating scientific investigations and accelerating discoveries in diverse fields, from basic biological research to drug development and diagnostics. Discover recombinant biosimilar antibodies from antibodies-online.com.
Biosimilar Research Antibodies for Top Selling Biologicals
Below you may find the main indications, mechanisms and matching monoclonal biosimilar research antibodies for the top selling biologicals:
-
Adalimumab
- Therapeutic Indications: Rheumatoid arthritis, psoriasis, Crohn's disease, ulcerative colitis, ankylosing spondylitis, and others.
- Mechanism: TNF-alpha inhibitor.
- Biosimilar Research Antibody: Adalimumab Biosimilar Research Antibody
-
Rituximab
- Therapeutic Indications: Non-Hodgkin lymphoma, chronic lymphocytic leukemia, rheumatoid arthritis.
- Mechanism: Monoclonal antibody targeting CD20 antigen.
- Biosimilar Research Antibody: Rituximab Biosimilar Research Antibody
-
Trastuzumab
- Therapeutic Indications: HER2-positive breast cancer, gastric cancer.
- Mechanism: Monoclonal antibody targeting HER2 receptor.
- Biosimilar Research Antibody: Trastuzumab Biosimilar Research Antibody
-
Pembrolizumab
- Therapeutic Indications: Various cancers, including melanoma, lung cancer, and others.
- Mechanism: Immune checkpoint inhibitor (PD-1 inhibitor).
- Biosimilar Research Antibody: Trastuzumab Biosimilar Research Antibody
-
Enbrel
- Therapeutic Indications: Rheumatoid arthritis, psoriasis, ankylosing spondylitis, and others.
- Mechanism: TNF-alpha inhibitor.
- Biosimilar Research Antibody:
-
Infliximab
- Therapeutic Indications: Rheumatoid arthritis, Crohn's disease, ulcerative colitis, psoriasis, ankylosing spondylitis.
- Mechanism: TNF-alpha inhibitor.
- Biosimilar Research Antibody: Infliximab Biosimilar Research Antibody
-
Bevacizumab
- Therapeutic Indications: Various cancers, including colorectal, lung, and kidney cancer.
- Mechanism: VEGF inhibitor.
- Biosimilar Research Antibody: Bevacizumab Biosimilar Research Antibody
-
Aflibercept
- Therapeutic Indications: Age-related macular degeneration, diabetic macular edema, macular edema following retinal vein occlusion.
- Mechanism: VEGF inhibitor.
- Biosimilar Research Antibody: Aflibercept Biosimilar Research Antibody
-
Nivolumab
- Therapeutic Indications: Various cancers, including melanoma, lung cancer, and others.
- Mechanism: Immune checkpoint inhibitor (PD-1 inhibitor).
- Biosimilar Research Antibody: Nivolumab Biosimilar Research Antibody
-
Daratumumab
- Therapeutic Indications: Multiple myeloma.
- Mechanism: Monoclonal antibody targeting CD38 antigen.
- Biosimilar Research Antibody: Daratumumab Biosimilar Research Antibody
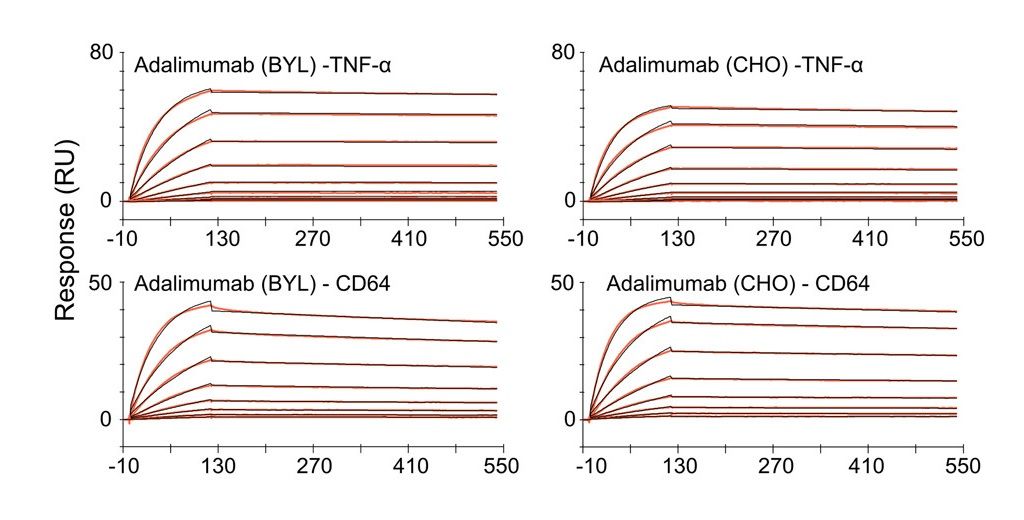
Case Study: Assessing Binding Affinity of Adalimumab Biosimilar
SPR analysis of adalimumab and its binding partners. Immobilized antibody molecules produced from either BYL or CHO cells were probed with recombinant TNFa and CD64 to show ligand and receptor binding, respectively.
Biosimilar Antibody used as benchmark: Recombinant TNF Alpha Antibody (ABIN5668145) .
Source: Biotech & Bioengineering, First published: 28 June 2023, DOI: (10.1002/bit.28461)
Biosimilar Antibodies in Drug Discovery
In drug discovery and subsequent development phases, biosimilar antibodies like Infliximab can be used as benchmarks in several ways:
- Analytical Methods and Characterization: Molecular structure, post-translational modifications (PTMs), binding-specifities, and other attributes of approved biologicals are well-characterized due to their history as a reference biologic. Analytical methods and characterization techniques for new biosimilars or alternative therapy approaches can be benchmarked against those used for existing biosimilars to ensure accurate assessment of similarity.
- Pharmacokinetics: Biosimilars can provide a reference point for comparing the pharmacokinetic properties of a biosimilar to those of the reference product. Pharmacokinetics refers to the study of how a drug is absorbed, distributed, metabolized, and eliminated by the body.
- Immunogenicity Assessment: Immunogenicity profiles, or the likelihood of triggering an immune response, are known from clinical experience for biosimilars that have already been introduced to the market. When developing a biosimilar or an alternative therapeutic approach, companies benchmark their immunogenicity assessment strategies against those used for existing biosimilars to ensure appropriate evaluation of potential immune reactions.
- Clinical Trial Design: Clinical trials for biosimilar development often include a comparative efficacy and safety study, where the biosimilar is compared to the established reference product (e.g. Trastuzumab). The clinical trial design, endpoints, patient populations, and statistical analyses are often compared to existing trials of the reference product.
- Regulatory Approval Pathways: Regulatory authorities, such as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), use approved biosimilars (e.g. Ranibizumab) as a reference when establishing guidelines and requirements for biosimilar approval. Developers of biosimilars can adapt their development processes and compare their data against the regulatory expectations set for existing biosimilars.
Overall, biosimilars that are already in the clinic have a well-established history, clinical data, and regulatory status that make them valuable benchmarks in drug discovery to demonstrate the similarity, safety, and effectiveness of new drug candidates. Established and well studied biosimilars serve as gold standard when establishing new biological drugs and treatments. antibodies-online.com offers a very broad range of biosimilars and a great product range to choose from.
Explore all Biosimilar Research Antibodies
Recommended next Filters:
您想试用我们的人工智能搜索吗?
1,822 results




